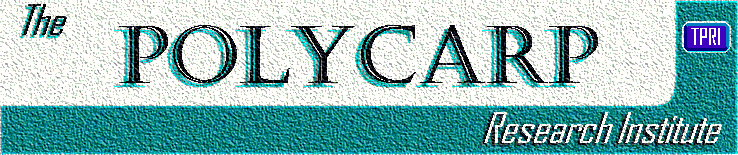
Español
Français
 View Printable PDF Version
View Printable PDF Version |
English Español Français |
 View Printable PDF Version View Printable PDF Version |
|
The Annals of Pharmacotherapy: Vol. 36, No. 3, pp. 465–470.
Postfertilization Effect of Hormonal Emergency Contraception
Chris Kahlenborn,
1 Joseph B Stanford, 2
and Walter L Larimore3
DATA SOURCES AND STUDY SELECTION: A MEDLINE search (1966–November 2001) was done to identify all pertinent English-language journal articles. A review of reference sections of the major review articles was performed to identify additional articles. Search terms included emergency contraception, postcoital contraception, postfertilization effect, Yuzpe regimen, levonorgestrel, mechanism of action, Plan B.
DATA SYNTHESIS: The 2 most common types of hormonal EC used in the US are the Yuzpe regimen (high-dose ethinyl estradiol with high-dose levonorgestrel) and Plan B (high-dose levonorgestrel alone). Although both methods sometimes stop ovulation, they may also act by reducing the probability of implantation, due to their adverse effect on the endometrium (a postfertilization effect). The available evidence for a postfertilization effect is moderately strong, whether hormonal EC is used in the preovulatory, ovulatory, or postovulatory phase of the menstrual cycle.
CONCLUSIONS: Based on the present theoretical and empirical evidence, both the Yuzpe regimen and Plan B likely act at times by causing a postfertilization effect, regardless of when in the menstrual cycle they are used. These findings have potential implications in such areas as informed consent, emergency department protocols, and conscience clauses.
OBJETIVO: Evaluar la posibilidad de un efecto de post-fertilización con relación a los tipos de contracepción hormonal de emergencia más comúnes utilizados en los EU, y explorar el impacto ético de esta posibilidad.
FUENTES DE INFORMACIÓN: Se realizó una búsqueda en MEDLINE del 1966 a noviembre 2001 con el propósito de identificar todos los artículos pertinentes en el idioma inglés. Una revisión de las secciones de referencia de los artículos de revisión principales se realizó para identificar artículos adicionales.
SÍNTESIS: Los tipos más comúnes de contracepción hormonal de emergencia utilizados en los EUson el régimen Yuzpe (dosis alta de etînil-estradiol con dosis alta de levonorgestrel) y Plan B (dosis alta de levonorgestrel sólo). Aunque ambos métodos en ocasiones detienen la ovulación, también podrían actuar reduciendo la posibilidad de implantación debido a su efecto adverso en el endometrio (un efecto de post-fertilización). La evidencia disponible para un efecto de post-fertilización es moderadamente fuerte, ya sea que se utilize la contracepción hormonal de emergencia en la fase pre-ovulatoria, ovulatoria, o post-ovulatoria del ciclo menstrual.
CONCLUSIONES: En base a la evidencia teórica y empírica presente, ambos el régimen Yuzpe y el Plan B, probablemente actúan en ocasiones causando un efecto de post-fertilización independientemente de cuándo, durante el ciclo menstrual, son utilizados. Estos hallazgos tienen implicaciones potenciales en tales áreas como el consentimiento educado, los protocolos de salas de emergencia y las cláusulas de consciencia.
Brenda R Morand
OBJECTIF: Évaluer les effets des médicaments permettant une contraception orale d'urgence sur la fécondation et discuter les répercussions éthiques de ces effets.
REVUE DE LITTÉRATURE ET SÉLECTION DES ÉTUDES: Recherche de la base de données MEDLINE (1966 à novembre 2001) des articles pertinents de langue anglaise et revue systématique de la bibliographie des articles identifiés.
RÉSUMÉ DES DONNÉES: Les deux régimes les plus fréquemment utilisés aux États-Unis pour la contraception orale d'urgence sont celui de Yuzpe (hautes doses d'éthinylestradiol et de lévonorgestrel) et celui du Plan B (hautes doses de lévonorgestrel). La principale action pharmacologique de ces 2 régimes semble être associée à leur pouvoir inihibiteur de l'ovulation. Cependant, il semble qu'ils aient aussi un effet au niveau de l'endomètre, interférant ainsi l'implantation de l'ovule fécondée. Plusieurs études font état de cette dernière action pharmacologique et ce, indépendamment de la période du cycle menstruel durant laquelle ces régimes sont donnés (phase pré- ou post-ovulatoire).
CONCLUSIONS: En plus d'inihiber l'ovulation, la contraception orale d'urgence semble altérer les propriétés de l'endomètre pouvant causer ainsi un avortement précoce. Considérant cet effet pharmacologique potentiel, l'administration de la contraception orale d'urgence soulève alors certains aspects éthiques quant à l'obtention d'un consentement éclairé de la patiente, quant à l'implantation systématique de protocoles dans les salles d'urgence et quant à la responsabilité éthique des professionnels de la santé prescrivant et administrant cette médication.
Sylvie Robert
KEY WORDS: contraception, levonorgestrel, postfertilization effect.
Ann Pharmacother 2002;36:465–470.
Emergency contraception (EC) consists of hormones or mechanical devices used within 72 hours of sexual intercourse with the intent of preventing pregnancy. In the late 1960s and early 1970s, women used high-dose estrogens such as diethylstilbestrol as EC.1 This treatment was replaced in 1974 by combination high-dose oral contraceptives (OCs) (e.g., ethinyl estradiol/levonorgestrel) used within a 12-hour interval (i.e., the Yuzpe regimen) and, in later years, by Plan B, which consists of 2 levonorgestrel tablets.2 The intrauterine device, danazol (danocrine), and mifepristone have also been studied and promoted as EC, but the Yuzpe regimen remains the most prevalent form of EC in the US and Europe.3
The question as to whether hormonal EC sometimes acts after fertilization to prevent implantation, called a postfertilization effect (i.e., early abortion), is important and could have far-reaching implications given the differing attitudes in regard to its use and related issues such as informed consent, emergency department rape protocols, and conscience clauses. Postfertilization effect refers to any effect that reduces the survival rate of the zygote/embryo after fertilization, usually prior to clinical recognition of pregnancy. We use the term early abortion synonymously with postfertilization effect. We recognize that some physicians,4 geneticists, and ethicists have arbitrarily defined human life as beginning after implantation, thereby eschewing the possibility of an early abortion prior to implantation. However, we recognize the traditional definition of pregnancy: “the gestational process, comprising the growth and development within a woman of a new individual from conception through embryonic and fetal period to birth,” where conception is defined as “the beginning of pregnancy, usually taken to be the instant that a spermatozoon enters an ovum and forms a viable zygote.”5
In a previous review6 of the mechanism of action of OCs, we concluded that they act at times via a postfertilization effect, that is, after fertilization and prior to the clinical recognition of pregnancy. However, the Yuzpe regimen and Plan B have a different dose and time course for use, which may result in different actions on the reproductive system. This article reviews data on the use of the Yuzpe regimen and Plan B with regard to their mechanisms of action and any potential ethical implications of those mechanisms.
Mechanisms of Action Return to Top
The Physicians' Desk Reference7 states: “ECPs (Emergency Contraceptive Pills) act primarily by inhibiting ovulation. They may act by altering tubal transport of the sperm and/or ova and/or altering the endometrium (thereby inhibiting implantation).” The Medical Letter2 ,8 stated in regard to hormonal EC: “Some studies have shown alteration in the endometrium, suggesting that they could also interfere with implantation of the fertilized egg, but other studies have found no such effects.” Therefore, the critical ethical questions are: Does use of the Yuzpe regimen or Plan B have a postfertilization effect; that is, does hormonal EC use at times cause an early abortion by altering the receptive properties of the endometrium? Can such an effect occur when EC is used in the preovulatory phase of the cycle, or does the postfertilization effect occur only when it is used in the ovulatory or postovulatory phase?
It is often asserted that hormonal EC use consistently stops ovulation. In an early study with oral contraceptives, Carr et al.9 found that a woman's estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone concentrations decreased significantly once she started using OCs. Because an LH surge is presumed necessary for ovulation, this result has been cited by many as evidence that hormonal contraceptive use completely inhibits ovulation. However, the findings of the Carr et al. article cannot be extrapolated to today's Yuzpe regimen or Plan B for several reasons. First, although the article was written in 1979, when the doses of estrogen in OCs were higher than the doses in today's OCs, using high-dose hormones in mid-cycle is far different from using them for 21 days in a 28-day cycle. Second, the findings were based on the results of only 4 ovulating women. Therefore, data from that study cannot be used to establish that use of today's OCs or hormonal EC consistently suppresses ovulation.
Further data from hormonal assays confirm that EC use does not consistently stop ovulation. One study10 of the Yuzpe regimen that examined serum hormonal markers of ovulation noted that an LH peak concentration occurred within 4 days after the treatment in 5 of 9 women, with a subsequent increase of progesterone, suggesting that ovulation had occurred. A more recent trial11 using urine hormonal markers found an LH peak concentration within 1 day of treatment with the Yuzpe regimen in 2 of 8 women, with a subsequent confirmatory increase of progesterone.
EFFECTS OF HORMONAL EC IN THE PREOVULATORY PHASE
Table 1 12–20 notes the major studies that have analyzed hormonal EC use. The estimated efficacy rates range from 56.9% to 90.9%, with the largest trial12 showing a 56.9% efficacy rate. The efficacy rate is the percent of reduction in the pregnancy rate of women who used hormonal EC compared with the estimated rate of women who did not. These rates are calculated from secondary data sources and have not been established via a randomized, controlled, prospective study (Appendix I3 ,20–28 ). This study,12 by the World Health Organization, found that in a group of about 400 women, 6 who used the Yuzpe regimen in the preovulatory phase became pregnant (10 were expected to become pregnant if no EC was used). In addition, 2 women who used Plan B in the preovulatory phase became pregnant (11 were expected). The preovulatory period is the time of the menstrual cycle that occurs more than 3 days prior to the expected day of ovulation. The expected day of ovulation in this study was estimated as the 14th day prior to the onset of the next menstrual cycle. Although this is an imprecise definition with the potential for significant misclassification, it is the best definition available for these studies. In addition, Glasier17 presented 2 cases of women who became pregnant after using the Yuzpe regimen while their progesterone concentrations were <1.5 ng/mL.
Therefore, at least 2 studies12 ,17 have shown that hormonal EC use, even in the preovulatory phase, does not consistently prevent pregnancy and, by definition, allows ovulation in those cases. Some have speculated29 that if ovulation is not inhibited, other mechanisms, such as a change in the viscosity of cervical mucus and/or an alteration in the tubal transport of sperm, ovum, or embryo, may come into play. However, there are no clinical data to address these theoretical mechanisms. In contrast, there are clinical data directly relevant to the potential effects of hormonal EC use on implantation.
OCs are known to adversely affect the implantation process,6 which has implications for the Yuzpe regimen and Plan B because they are composed of the same (or similar) hormones contained in today's OCs. OCs affect integrins, a group of adhesion molecules that have been implicated as playing an important role in the area of fertilization and implantation. Somkuti et al.30 noted: “These alterations in epithelial and stromal integrin expression suggest that impaired uterine receptivity is one mechanism whereby OCs exert their contraceptive action.” In addition, prostaglandins are critical for implantation, but OC use lowers uterine prostaglandin concentrations.31 ,32 Finally, it is well known that OC use decreases the thickness of the endometrium as verified by magnetic resonance imaging scans,33 ,34 and a thinner endometrium makes implantation more difficult.35–39 Because hormonal EC consists of hormones contained within OCs, it is possible that the use of hormonal EC has some of the same effects on the endometrium as does the use of OCs. A number of studies support this hypothesis, noting changes in endometrial histology,1 ,40 or uterine hormone receptor levels41 that persist for days after women used the Yuzpe regimen. All of these findings imply that use of the Yuzpe regimen unfavorably alters the endometrium.
In addition to the theoretical evidence that EC use adversely affects implantation, Hertzen and Van Look12 found that both use of the Yuzpe regimen and Plan B reduced the expected number of pregnancies when they were used in the ovulatory phase (17–13 d prior to the next menstrual cycle) and postovulatory phase (13 d prior to the expected menstrual cycle), as well as in the preovulatory phase (as discussed earlier). In the groups that used the Yuzpe regimen in the ovulatory phase, 17 pregnancies occurred (54 were expected if EC was not used), whereas 7 occurred in the postovulatory phase (11 were expected). In the group that used Plan B, 7 pregnancies occurred (53 were expected) in the ovulatory phase, whereas 2 occurred in the postovulatory phase (10 were expected). These data are highly consistent with the hypothesis that hormonal EC has a postfertilization effect on the endometrium. In the case of the use of hormonal EC in the ovulatory phase, it is still possible that other mechanisms might come into play (i.e., a change in the viscosity of cervical mucus and/or an alteration in the tubal transport of either the sperm, ovum, or embryo). However, we could find no data to support these theories.
Increased Risk of Ectopic Pregnancy? Return to Top
One result of a postfertilization effect of hormonal EC use might be an increased proportion of recognized pregnancies that are ectopic. If the actions of hormonal EC on the fallopian tube and endometrium were such as to have no postfertilization effects, then the reduction in the rate of intrauterine pregnancies (IUPs) in women taking agents used in EC should be proportional to the reduction in the rate of extrauterine pregnancies (EPs) in women using hormonal EC. However, if the effect of hormonal ECs is to increase the EP/IUP ratio, this would indicate that one or more postfertilization effects are operating.6
The current proportion of clinical pregnancies that are ectopic is a little less than 2%.42 In the only study that we are aware of regarding hormonal EC and ectopic pregnancy, Kubba and Guillebaud43 noted that in 715 women who used the Yuzpe regimen, 17 pregnancies occurred, including 1 ectopic pregnancy (i.e., a 5.9% rate of ectopic pregnancy), supporting the possibility of one or more postfertilization effects. However, the confirmation of a postfertilization effect would take a much larger series of hormonal EC pregnancies to determine whether the proportion of ectopic pregnancies is indeed higher than in those not having used EC.
Relative Contribution of Postfertilization Effect Return to Top
As noted earlier, 2 small studies10 ,11 have suggested that when EC is used before ovulation, ovulation may be inhibited in 55–75% of the cases. Under the highly optimistic assumption that hormonal EC use prevents ovulation in 87.5% of women treated, Trussell and Raymond44 estimated that a mechanism “other than preventing ovulation accounts for 13–38% of the estimated effectiveness of the Yuzpe regimen.” This range is higher than 12.5% because hormonal EC is often used during or after ovulation when, by definition, mechanisms other than prevention of ovulation are in effect. The most likely candidate for the mechanism “other than preventing ovulation” is a postfertilization effect (by effects on the endometrium).
Summary and Implications Return to Top
The evidence to date supports the contention that use of EC does not always inhibit ovulation even if used in the preovulatory phase, and that it may unfavorably alter the endometrial lining regardless of when in the cycle it is used, with the effect persisting for days. The reduced rates of observable pregnancy compared with the expected rates in women who use hormonal EC in the preovulatory, ovulatory, or postovulatory phase are consistent with a postfertilization effect, which may occur when hormonal EC is used in any of these menstrual phases.
This interpretation of the cited literature has important ramifications, given the polarizing opinions about EC use.45 For example, many state laws contain conscience clauses in which medical personnel (e.g., physicians, pharmacists, nurses, physician assistants, nurse practitioners) cannot be forced to participate in, or refer for, any surgical or drug-induced abortions. Therefore, evidence in favor of a postfertilization effect may have legal implications for healthcare providers who either prescribe or have objections to prescribing these agents.
Emergency department protocols could also be impacted by evidence of a postfertilization effect. For example, emergency departments of Catholic hospitals usually allow either no use of hormonal EC in their rape protocols or limited use (i.e., preovulatory use of hormonal EC).45 Catholic hospitals that do allow hormonal EC use prior to ovulation may wish to reassess their policies given the findings that EC use does not consistently stop ovulation and has the potential of causing a postfertilization effect even when used prior to ovulation. Most large secular hospitals have fewer limitations on the use of hormonal EC as part of their rape protocols. Nevertheless, evidence of a postfertilization effect from use of hormonal EC is important to physicians who must make a moral decision about prescribing or referring for a drug that can cause an early abortion.
There are potential limitations in our conclusions. Because no controlled trials have been done with women using EC, our conclusions are based on the existing data of case series with historical controls. However, these are the best available data for hormonal EC use. In addition, we have assumed, based on our discussions with physicians and laypeople across the country, that a significant number of physicians and patients would be concerned about a possible postfertilization effect. Although some evidence does exist to support our assumption,45 ,46 further research is needed. Nevertheless, the principle of informed consent would state that it is important to inform women who may use hormonal EC about this possible effect so that they can choose based on the best available data.
Regardless of the personal beliefs of the physician or provider about the mechanism of hormonal EC use, it is important that patients have information relevant to their own beliefs and value systems. It has been suggested to us by some that postfertilization loss attributed to hormonal EC use would not need to be included in informed consent until it is either definitely proven to exist or proven to be a common event. However, rare but important events are an essential part of other informed-consent discussions in medicine, primarily when the rare possibility would be judged by the patient to be important. For example, anesthesia-related deaths are rare for elective surgery; nevertheless, it is considered appropriate and legally necessary to discuss this rare possibility with patients before such surgery because the possibility of death is so important. Therefore, for women to whom the induced death of a zygote/embryo is important, failure to discuss the possibility of this loss, even if the possibility is judged to be remote, would be a failure of informed consent. Furthermore, based on the data reviewed in this article, it seems that a postfertilization effect is probably more common than is recognized by most physicians or patients. This is particularly true because in the studies done to date, women have been more likely to request treatment after intercourse that occurred near the time of ovulation than after intercourse that occurred earlier in the cycle.44
Some have suggested to us that an overemphasis of possible postfertilization effects might make women choose not to use EC and therefore increase the incidence of unplanned pregnancies. Both of these views fail to acknowledge the value of a woman's right to make decisions based on informed consent. During informed-consent discussions, overemphasis of any single possible risk may not result in appropriate informed consent; however, failing to mention a possible risk would be a failure of adequate informed consent. Therefore, discussion of a potential postfertilization risk should occur and should be kept within the perspective of the available medical evidence.
Proper informed consent requires patient and physician comprehension of information, the disclosure of that information, and the sharing of interpretations. If a postfertilization mechanism of hormonal EC use violates the morals of any woman, the failure of the physician or care provider to disclose that information would effectively eliminate the likelihood that the woman's consent was truly informed.
Finally, there is in our view a potential for negative psychological impact on women who value human life from conception onward, and have not been given informed consent about hormonal EC use, and later learn of the potential postfertilization effects. Their responses could include disappointment, guilt, sadness, anger, rage, depression, or a sense of having been violated by the provider. To assume that all patients will not care about a postfertilization effect is not supported by the literature.45 ,47–49
Acknowledgment—I thank Dorothy Dugandzic and Walt
Severs for their technical and editorial assistance.
1. Yuzpe AA, Thurlow HJ,
Ramzy I, Leyshon JI. Post coital contraception — a pilot study. J
Reprod Med 1974;13:53–8. [PubMed
Citation]
2. Plan B: a progestin only
contraceptive. Med Lett 2000;42:10
3. LaValleur J. Emergency
contraception. Obstet Gynecol Clin North Am 2000;27:817–39. [PubMed
Citation]
4. Hughes EC, ed. Committee on
terminology, American College of Obstetricians and Gynecologists.
Obstetric–gynecological terminology Philadelphia: FA Davis. 1972
5. Mosby's medical, nursing, &
allied health dictionary. 6th ed. Philadelphia: Mosby. 2002
6. Larimore WL,
Stanford J. Postfertilization effects of oral contraceptives and their
relationship to informed consent. Arch Fam Med 2000;9:126–33. [PubMed
Citation]
7. Physicians' desk reference. 54th
ed. Montvale, NJ: Medical Economics. 2000:1335
8. An emergency contraceptive kit.
Med Lett 1998;40:102–3.
9. Carr BR, Parker CR,
Madden JM, MacDonald PA, Porter JC. Plasma levels of
adrenocorticotropin and cortisol in women receiving oral contraceptive steroid
treatment. J Clin Endocrinol Metab 1979;49:346–9. [PubMed
Citation]
10. Ling WY, Robichaud A,
Zayid I, Wrixon W, MacLeod SC. Mode of action of dl-norgestrel
and ethinylestradiol combination in postcoital contraception. Fertil Steril
1979;32:297–302. [PubMed
Citation]
11. Swahn LM, Westlund P,
Johannisson E, Bygdeman M. Effect of post-coital contraceptive
methods on the endometrium and the menstrual cycle. Acta Obstet Gynecol Scand
1996;75:738–44. [PubMed
Citation]
12. Hertzen H, Van
Look PFA. Randomised controlled trial of levonorgestrel versus the Yuzpe
regimen of combined oral contraceptives for emergency contraception. Lancet
1998;352:428–33. [PubMed
Citation]
13. Webb AMC, Russell J,
Elstein M. Comparison of Yuzpe regimen, danazol, and mifepristone (RU 486)
in oral postcoital contraception. BMJ 1992;305:927–31.
14. Zuliani G,
Colombo UF, Molla R. Hormonal postcoital contraception with an
ethinylestradiol–norgestrel combination and two danazol regimens. Eur J Obstet
Gynecol Reprod Biol 1990;37:253–60. [PubMed
Citation]
15. Yuzpe AA, Smith RP,
Rademaker AW. A multicenter clinical investigation employing ethinyl
estradiol combined with dl-norgestrel as a postcoital contraceptive agent.
Fertil Steril 1982;37:508–13. [PubMed
Citation]
16. Ho PC, Kwan MSW. A
prospective randomized comparison of levonorgestrel with the Yuzpe regimen in
post-coital contraception. Hum Reprod 1993;8:389–92. [PubMed
Citation]
17. Glasier A, Thong KJ,
Dewar M, Mackie M, Baird DT. Mifepristone (RU-486) compared with
high-dose estrogen and progestogen for emergency postcoital contraception. N
Engl J Med 1992;327:1041–4. [PubMed
Citation]
18. Van Santen MR,
Haspels AA. A comparison of high-dose estrogens versus low-dose
ethinylestradiol and norgestrel combination in postcoital interception: a study
in 493 women. Fertil Steril 1985;43:206–13. [PubMed
Citation]
19. Percival-Smith RK,
Abercrombie B. Postcoital contraception with dl-norgestrel/ethinyl
estradiol combination: six years experience in a student medical clinic.
Contraception 1987;36:287–93. [PubMed
Citation]
20. Trussell J,
Rodriguez G, Ellertson C. Updated estimates of the effectiveness of
the Yuzpe regimen of emergency contraception. Contraception 1999;59:147–51. [PubMed
Citation]
21. Glasier A. Emergency
contraception. Br Med Bull 2000;56:729–38. [PubMed
Citation]
22. Dixon GW,
Schlesselman JJ, Ory HW, Blye RP. Ethinyl estradiol and
conjugated estrogens as postcoital contraceptives. JAMA 1980;244:1336–9. [PubMed
Citation]
23. Schwartz D,
Mayaux MJ, Martin-Boyce A, Czyglik F, David G. Donor
insemination: conception rate according to cycle day in a series of 821 cycles
with a single insemination. Fertil Steril 1979;31:226–9. [PubMed
Citation]
24. Barrett JC,
Marshall J. The risk of conception on different days of the menstrual
cycle. Popul Stud 1969;23:455–61.
25. Wilcox AJ,
Weinberg CR, Baird DD. Timing of sexual intercourse in relation to
ovulation. Effects on probability of conception, survival of the pregnancy, and
sex of baby. N Engl J Med 1995;333:1517–21. [PubMed
Citation]
26. Hacker NF, Moore JG,
Essentials of obstetrics and gynecology. 3rd ed. Philadelphia: WB Saunders.
1998
27. Diamond EF. Ovral in rape
protocols. Ethics Medics 1996;2110:2
28. Wilcox AJ, Dunson D,
Baird DD. The timing of the “fertile window” in the menstrual cycle: day
specific estimates from a prospective study. BMJ 2000;321:1259–62.
29. Glasier A. Emergency
postcoital contraception. N Engl J Med 1997;337:1058–64. [PubMed
Citation]
30. Somkuti SG, Sun J,
Yowell C, Fritz M, Lessey B. The effect of oral contraceptive
pills on markers of endometrial receptivity. Fertil Steril 1996;65:484–8. [PubMed
Citation]
31. Dawood YM, Ibuprofen and
dysmenorrhea. Am J Med 1984;771 A.:87–94.
32. Bieglmayer C,
Hofer G, Kainz C, Reinthaller A, Kopp B, Janisch H.
Concentration of various arachidonic acid metabolites in menstrual fluid are
associated with menstrual pain and are influenced by hormonal contraceptives.
Gynecol Endocrin 1995;9:307–12. [PubMed
Citation]
33. Brown HK, Stoll BS,
Nicosia SV, Fiorica JV, Hambley PS, Clarke LP. Uterine
junctional zone: correlation between histiologic findings and MR imaging.
Radiology 1991;179:409–13. [PubMed
Citation]
34. Demas BE, Hricak H,
Jaffe RB. Uterine MR imaging: effects of hormonal stimulation. Radiology
1986;159:123–6. [PubMed
Citation]
35. Abdalla HI,
Brooks AA, Johnson MR, Kirkland A, Thomas A, Studd JW.
Endometrial thickness: a predictor of implantation in ovum recipients?. Hum
Reprod 1994;9:363–5. [PubMed
Citation]
36. Dickey RP, Olar TT,
Taylor SN, Curole DN, Matulich EM. Relationship of endometrial
thickness and pattern to fecundity in ovulation induction cycles: effect of
clomiphene citrate alone and with human menopausal gonadotropin. Fertil Steril
1993;59:756–60. [PubMed
Citation]
37. Gonen Y, Casper RF,
Jacobson W, Blankier J. Endometrial thickness and growth during ovarian
stimulation: a possible predictor of implantation in in-vitro fertilization.
Fertil Steril 1989;52:446–50. [PubMed
Citation]
38. Schwartz LB, Chiu AS,
Courtney M, Krey L, Schmidt-Sarosi C. The embryo versus
endometrium controversy revisited as it relates to predicting pregnancy outcome
in in-vitro fertilization — embryo transfer cycles. Hum Reprod 1997;12:45–50. [PubMed
Citation]
39. Shoham Z, Carlo C,
Patel A, Conway GS, Jacobs HS. Is it possible to run a succesful
ovulation induction program based solely on ultrasound monitoring: the
importance of endometrial measurements. Fertil Steril 1991;56:836–41. [PubMed
Citation]
40. Ling WY, Wrixon W,
Zayid I, Acorn T, Popat R, Wilson E. Mode of action of
dl-norgestrel and ethinylestradiol combination in postcoital contraception. II.
Effect of postovulatory administration on ovarian function and endometrium.
Fertil Steril 1983;39:292–7. [PubMed
Citation]
41. Kubba AA, White JO,
Guillebaud J, Elder MG. The biochemistry of human endometrium after
two regimens of postcoital contraception: a dl-norgestrel/ethinylestradiol
combination or danazol. Fertil Steril 1986;45:512–6. [PubMed
Citation]
42. Aboud A. A five-year
review of ectopic pregnancy. Clin Exp Obstet Gynecol 1997;24:127–9. [PubMed
Citation]
43. Kubba AA,
Guillebaud J. Case of ectopic pregnancy after postcoital contraception
with ethinyloestradiol–levonorgestrel. Br Med J 1983;287:1343–4. [PubMed
Citation]
44. Trussell J,
Raymond EG. Statistical evidence about the mechanism of action of the
Yuzpe regimen of emergency contraception. Obstet Gynecol 1999;93:872–6. [PubMed
Citation]
45. Golden NH, Seigel WM,
Fisher M, Schneider M, Quijano E, Suss A. Emergency
contraception: pediatricians' knowledge, attitudes and opinions. Pediatrics
2001;107:287–92. [PubMed
Citation]
46. Spinnato JA. Mechanism of
action of intrauterine contraceptive devices and its relation to informed
consent. Am J Obstet Gynecol 1997;176:503–6. [PubMed
Citation]
47. Wilkinson J, Ethical
problems at the beginning of life. In: Wilkinson J, ed. Christian ethics in health
care: a source book for Christian doctors, nurses and other health care
professionals Edinburgh, Scotland: Handsel Press. 1988:176–208.
48. Ryder RE. Natural family
planning: effective birth control supported by the Catholic Church. BMJ
1993;307:723–6.
49. Tonti-Filippini N, The
pill: abortifacient or contraceptive: a literature review. Linacre Quarterly
1995 Feb.:5–28.
APPENDIX Return to Top
The measure of efficacy is critical to an analysis of a possible postfertilization effect. For example, if hormonal EC use had a 0% efficacy rate, the question of a postfertilization effect would be irrelevant. Hormonal EC use received Food and Drug Administration approval without evidence of a randomized, controlled, prospective study regarding its effectiveness.3 ,21 Rather, effectiveness was estimated based on the studies we have reviewed in this article. We noted the efficacy rates based on the raw data versus Trussell et al.'s20 calculated estimates for each of the 8 trials presented in Table 1 . Trussell et al. used the latter estimates to calculate an overall efficacy rate of 74.1%, while the raw data yield a figure of 65.7%.
In these studies, the pregnancy rates of the cohort were compared with pregnancy rates estimated from historical controls. Specifically, the control pregnancy rates were based on the procedure developed in the Dixon Study,22 which estimated the expected rate of pregnancy in women from a single act of intercourse on a particular day of the menstrual cycle. Dixon based the probability of pregnancy per specific day on 2 major studies: Schwartz et al. (1979)23 and Barrett and Marshall (1969).24 In subsequent analyses, Trussell et al. dropped the Schwartz study, which was based on artificial insemination, and added another historical control group from a cohort of women trying to achieve pregnancy in North Carolina in the early 1980s.25 In doing this, Trussell et al. were in fact not comparing contemporaneous cohorts and controls. This major design problem may render the conclusions of the studies uncertain for 2 reasons:
1. In the 1960s, the rate of infertility was lower than in later years. For example, “infertility increased 177% among married women aged 20 to 24 years between 1965 and 1982.”26 Therefore, the rate of infertility would be expected to be lower for the Barrett controls than for the study cohorts (women using EC). In addition, Wilcox et al.25 noted that “women were excluded if they had a serious chronic illness or if they or their partners had a history of fertility problems.” None of the case studies reported specifically screening for infertility. It is therefore probable that both of the historical control studies had a lower rate of infertility than the case studies. If this is true, then studies of EC use that employ historical controls for comparison may overestimate the effectiveness of EC use in preventing or ending a pregnancy.
2. Selecting controls from women who were not seeking to use EC to avoid pregnancy may lead to differences that could affect the results. For example, some controls came from the Barrett and Marshall study,24 which examined 241 couples who were using a natural family planning method based on basal body temperature. Some of these women were trying to conceive, as were the women enrolled in the Wilcox et al. trial.25 None was known to be under the stress of a rape or other high-stress situation. However, the cohort in the 8 trials cited by Trussell were trying to prevent or end their pregnancy and were probably under more emotional stress than the controls who desired pregnancy. If 2 groups of women are examined, one that desires pregnancy and the other that does not and is under stress, the fertility rates in each group may vary markedly because it is possible that under extreme stress, the secretion of ovulatory hormones from the pituitary gland could be inhibited. For example, Diamond27 noted a prospective study in Minnesota of 4000 women who had been raped and none had become pregnant. This may reflect an endogenous hormonal change whereby the women's bodies inhibited ovulation during or shortly after the time of the sexual assault.
3. All of the EC studies are based on a fixed timing of ovulation relative to cycle length (e.g., 14 d before the next menstrual cycle). However, the length of the luteal phase varies significantly, both between women, and to a lesser extent, within the same woman, even for women of regular cycles.28 Therefore, the assignment of conception probabilities based on day relative to ovulation is imprecise.
We believe for
the above-noted reasons that the estimates of efficacy rates for hormonal EC
use are highly tentative and require further analysis.
1Chris Kahlenborn MD,
Department of Internal Medicine, Altoona Hospital, Altoona, PA; Department of
Internal Medicine, Bon Secour Hospital, Altoona, E-mail: kahlen@alt3.com
2Joseph B Stanford MD
MSPH, Assistant Professor, Department of Family and Preventive Medicine,
University of Utah, Salt Lake City, UT
3Walter L Larimore MD,
Associate Clinical Professor, Community and Family Medicine, University of
South Florida, Tampa, FL; Vice-President, Medical Outreach, Focus on the
Family, Colorado Springs, CO.
4Reprints available from The Annals of
Pharmacotherapy
Table 1. Major Studies on Efficacy Rates of the Yuzpe Regimen of Emergency Contraception