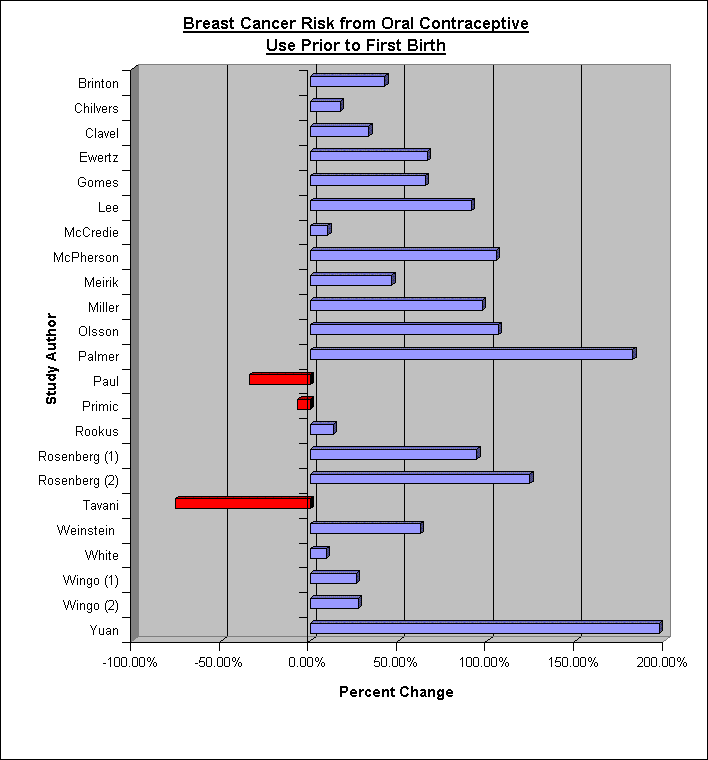
As of 2003, 18 out of 21 retrospective studies show that women who take oral contraceptives prior to their first-term birth incur an increased risk in developing breast cancer as noted in the bar graph below.

 View Printable PDF Version (article below) View Printable PDF Version (article below) |
Overview:
Breast Cancer and the Pill
Q-A: What is an oral contraceptive pill?
An
oral contraceptive pill is usually a combination of a synthetic estrogen
and progestin (ie, the two major types of female hormones) which women
take for 21 days out of a 28-day cycle. These hormones work by suppressing, but
not eliminating ovulation, thickening cervical mucus, and by changing the
lining of the uterus.
Q-B: Is there any evidence that
OCP (oral contraceptive pill) use causes breast cancer in animals?
Yes.
Concerns were raised in 1972 when it was noted that an oral contraceptive pill
containing the artificial hormones mestranol and norethynodrel appeared to
cause a case of metastatic breast cancer in a group of six female rhesus
monkeys [1]. This was especially worrisome because rhesus monkeys rarely
develop breast cancer. Until that time, only three cases of breast cancer in
rhesus monkeys were reported. Although some argued that this was simply a
“chance finding,” concern grew further when it was noted that both beagles and
rodents developed breast cancer when exposed to the hormones contained in
today’s OCPs [sources: 2, 3, 4, 5, 6].
Q-C: How might OCP use cause breast cancer in humans?
In 1989, Anderson et al [7]
published a classic paper regarding the influence of OCP use on the rate of
breast cell division. They found that nulliparous women (ie, women who
have not had children) who took OCPs had a significantly higher rate of breast
cell division than nulliparous women who did not take them. This was especially
important because it is known that in general, cells that divide more rapidly
are more vulnerable to carcinogens (ie, cancer producing agents) and
thus more likely to become cancerous.
Q-D: Does OCP use cause an early abortion and if so, could this also be playing a role in the increased risk of breast cancer?
Both pro-life and pro-abortion
groups openly admit that OCP use causes early abortions, with the latter doing
so publicly in testimony before the Supreme Court in 1989 [8]. Induced
abortion before a woman’s first full-term pregnancy (FFTP) has been
noted to increase a woman’s risk of breast cancer by 50% [9]. Could an abortion
(defined to be the death of the zygote, embryo or fetus after conception has
occurred) within the first week after conception have a deleterious effect as
concerns breast cancer? The hormonal physiology of early pregnancy is difficult
to measure but Stewart et al [10] and Norman et al [11] have shown that estradiol
and progesterone levels (ie, the female hormones) start to rise above
baseline levels within 4 days of conception, thus prior to implantation and
before hCG levels begin to rise. An early abortion would cause a sudden fall in
the levels of these hormones. Could this early “hormonal blow” be playing a
role? To this author’s knowledge, no one has asked or studied this question.
Q-E: Can you give a brief history of the studies that showed a link between OCP use prior to a first full-term pregnancy (FFTP) and the increased risk of breast cancer?
In 1981, Pike et al [12] found
that women who took OCPs for 4 years before their first full-term
pregnancy (FFTP) had at least a
2.25-fold (125%) increased risk of developing breast cancer before the age of
32. This startled the research world and led to additional studies, including a
very large American trial called the CASH study (ie, Cancer And Steroid Hormone
study). In 1993, the CASH study showed that women who took OCPs prior to their
FFTP and were under 44 years of age had a 40% increased risk of breast cancer,
which reached statistical significance in the 35 to 44 year-old age group [13].
Later in England, Chilvers et
al [14] published the results of another large study called the United Kingdom
National Study. She showed that young women under the age of 36 who had used
oral contraceptives for at least 4 years before their FFTP had at least a 44%
increased risk in breast cancer. The last large study was performed in 1995 by
Brinton et al [15]. It showed a 42% increased risk for women who used OCPs for
more than 6 months prior to their FFTP.
Q-F: If the major studies showed the risks that have been mentioned, then why do doctors and pharmacists fail to inform their patients of those risks?
That is a good question. Major
journals and major medical associations (eg, the AMA [American Medical
Association], the ACOG [American College of Obstetricians and Gynecologists],
and the AAP [American Academy of Pediatrics]) have failed to stress or properly
note this risk. Part of the problem is that because the OCP/breast cancer
debate is complicated, most people have to rely on what “the experts” tell
them.
A good example of this
occurred recently in the Oxford study reported in a condensed version in The Lancet [16] and in complete form in
Contraception [17]. This study was
and remains the largest meta-analysis (ie, a synthesis of all the major
studies done in a particular field, concluding in an overall risk for the
pooled studies) regarding the studies of OCPs and breast cancer. Researchers
from around the world studied and combined the data from 54 studies, involving
25 countries and 53,297 women who had breast cancer. It concluded that: “Women who are currently using combined oral
contraceptives or have used them in the past 10 years are at a slightly
increased risk of having breast cancer diagnosed, although the additional
cancers tend to be localized to the breast. There is no evidence of an increase
in the risk of having breast cancer diagnosed 10 or more years after cessation of use. . .” Unfortunately, this study is known more for what
it did say, than what it did not say! There were several major weaknesses of the study.
Q-G: What are the weaknesses of the Oxford study and what
implications do they have?
The main weakness was the failure to report any evidence of what
the pooled risk of oral contraceptive use before a first term pregnancy was in
women less than 45 years old. Another major weakness is that the Oxford
study pooled data from studies which looked at women with breast cancer from
the early and mid 1970s [17, p.5S].
A woman’s breast is especially
sensitive to carcinogenic influence (ie, cancer producing influence) before she has her first child because
the breast undergoes a maturing process throughout a woman’s first pregnancy.
By failing to measure the effect of OCP use before
a premenopausal woman’s first full-term pregnancy (FFTP), the Oxford study
failed to give data on the one group of women who are most likely to get breast
cancer from oral contraceptives, namely, those women who used them before their
FFTP (eg, many teenagers and women in their 20s).
The second weakness is that
the Oxford study used data from older studies which took some of their data
from the mid and early 1970s. This does not leave a long enough latent period.
A latent period is the time between exposure to a suspected risk factor
(eg, early OCP use) and the cancer which it increases (eg, breast cancer).
Often the latent period between a risk factor and a cancer is 15 to 20 years or
more (eg, cigarettes and lung cancer). Although women in the U.S. began taking
OCPs in the 1960s, they only began taking them for longer periods of time at
younger ages in the 1970s. Thus, only studies which include data from the 1980s
and 1990s or beyond would allow a long enough latent period to pick up the
influence of early OCP use.
Q-H: Why is it important to study women who are under the age of 45?
Women who are under the age of
45 are more likely to have used OCPs prior to having a child than woman over
the age of 45. For example a 55-year old woman who had breast cancer in 1990
would have been very unlikely to have taken the OCP for a significant period of
time prior to giving birth because OCPs were just coming to the U.S. in the
early 1960s when the cited woman would have been in her late 20s.
Q-I: What do the four largest studies, which take the bulk of their data after 1980, state regarding women who used OCPs prior to their first full-term pregnancy (FFTP)?
Table
3A: RISK OF BREAST CANCER TO WOMEN WITH OCP USE PRIOR TO their FFTP
AUTHOR YEARS STUDIED SIZE
OF STUDY FINDINGS
Wingo
[13] 12/80-82 2089 less than age 45 40% increase; ages 20-44
Rosenberg
[18] 1977-1992 1427 less
than age 45 88% increase*
White [19] 1983-1990 747 less
than age 45 50%
increase: use 5 years of menarche
Brinton [15] 5/90-12/92 1648 less than age 45 42%
increased risk*
*Computed from data from study, increase reflects the odds ratio.
The four largest retrospective
studies** of parous women under the age of 45 all show at least a 40% increased risk for women who took OCPs prior to
their FFTP or within 5 years of menarche. Two studies (Rosenberg and Brinton)
did not list a formal risk but it was calculated from the data in their paper.
**An example of a retrospective study is one in which women with breast cancer would be interviewed and asked questions about their risk factors such as family history, OCP use, induced abortion, etc.
Q-J: Has anyone done a meta-analysis of retrospective studies that examined the question of risk to women under the age of 45 who had taken OCPs prior to their FFTP?
Yes. Two different researchers
have addressed this question. Thomas et al, in 1991, found that women who took
OCPs for extended periods of time prior to their FFTP had a 44% increased risk
[RR=1.44 (1.23-1.69)] [20]. A more
refined meta-analysis in 1990 by Romieu et al restricted her analysis to those
studies done after 1980. The study showed that women under the age of 45 who
had taken OCPs for 4 or more years prior to their FFTP had a 72% increased
incidence [RR=1.72 (1.36-2.19)] of
breast cancer [21].
Q-K: Can you comment on why a recent large study published by researchers at Harvard claimed to show no increased risk of developing breast cancer in women who had taken OCPs for 5 years or more prior to their FFTP?
In 1997, a group of researchers
at Harvard Medical School led by Dr. Hankinson published a study in Cancer Causes and Control [22]. It based
its conclusions on data taken from the Nurses’ Health Study and claimed to show that women who took oral
contraceptive pills for 5 years or more prior to their FFTP had no increased
risk of developing breast cancer compared to women who never took OCPs [RR=0.57 (0.24-1.31)]. The study’s
conclusions appear to have been based on a flawed analysis.
Q-L: Can you describe the problems with the study?
Yes. The researchers compared
women with breast cancer who took OCPs for 5 years or more prior to their FFTP
[let’s refer to these women as Group A] to
women with breast cancer who never took OCPs [Group B].
It is known that women took
OCPs for longer periods of time and earlier in their reproductive lives
in the 1980s and 1990s than in the 1960s and 1970s as was clearly noted in the
Oxford study [17, p.9S; Tables 14, 15]. So any group of women who had taken
OCPs for 5 years or more prior to their FFTP (ie, Group A) would have been more likely to have done so
while in their late teens and 20s in the 1980s or 1990s, whereas women in
Group B (who never took OCPs) would be more likely to contain a
distribution of women who would have been in their late teens and 20s in either
the 1960s, 1970s, 1980s or 1990s. But this strongly supports the contention
that women in Group A would have a lower average age and a shorter follow-up
time than the women in Group B, which would of course invalidate the study’s
conclusions.
It is frightening to note that
the Harvard team presented no data on either the average age of women in the
noted groups or their respective lengths of follow-up time. The research team
instead chose to follow the noted groups in “person-years” as their measure of follow-up time.
This is the length of a follow-up period derived from the number of women
followed, multiplied by the average number of years they were followed. For
example, if group A had 100 women who were followed for 10 years, the total amount
of follow-up time would be 100 x 10 = 1,000 person-years. But if group A had
250 women who were followed for 4 years it would also have 1,000 person-years
of follow-up. This is totally inadequate because the measure of “person-years”
gives no data on the length of follow-up time in actual years and without this information the study must remain
suspect because it was noted that women
in group A most likely had both a younger average age and were followed for a
shorter period of time than the women in group B.
Q-M: Is there any way that the public will ever have access to the necessary data that was not presented in the Harvard study?
I am not sure. This author
tried in vain to obtain the answers to three basic questions over a 6 month
period of time from three different researchers involved in the Harvard study
via e-mail, phone calls and certified mail. It is ironic that one cannot access
data from these researchers especially because their study obtained its data
from the Nurses’ Health Study, a study
which was funded by citizen tax dollars through a grant via the NCI
(National Cancer Institute). The essential questions that need to be answered
are presented at the end of this chapter. If the Harvard team had answered
these questions the average age and follow-up time period for both Group A and
Group B’s women could have been easily calculated. Until the noted researchers
at Harvard make their data available for
all to see, the study’s conclusions must remain suspect.
Q-N: Have any other recent studies had methodological problems?
Yes, a large prospective study
conducted in England by Beral et al [23] claimed that a “cohort” (ie,
the group being examined in a prospective study) of 23,000 women who took the
OCP had no greater risk of developing breast cancer than 23,000 women who did
not take it. The main problem with the study is that women entered it from 1968
to 1969. But many of these women were taking OCPs after they had a FFTP because as we noted earlier, women took OCPs for shorter periods of time and later in their reproductive lives in
the 1960s and 1970s than in the 1980s and 1990s [17]. The study’s claim that
OCP use had no long-term risk of increasing breast cancer cannot be applied to
the subset of women who took (or currently take) OCPs for longer periods of
time prior to their FFTP.
Q-O: Can you give an overall statement regarding early OCP use and breast cancer?
Yes. If a woman takes the oral
contraceptive pill before her FFTP, she suffers a 40% increased risk of
developing breast cancer compared to women who do not take OCPs. If she takes
OCPs for 4 years or more prior to her FFTP, she may have an even higher risk,
as noted by Dr. Romieu earlier.
Q-P: Are any other groups of women at high risk?
Yes. Women who take OCPs for long periods of time (ie, 4 years or
more) [14,24,25], are at increased risk for developing breast cancer. Other
women at risk are those who use them
after the age of 25 [26,27,28] and nulliparous
women who use them for a long time (ie, 4 or more years) [14,29]. All three
categories of women seem to be at increased risk, with individual studies
ranging from 40% to over 200% increased risk. Women who took OCPs for longer
time periods and started using them at an early age appear to be at an even
greater risk. For example, the Brinton
study [15] is significant in that it allowed a longer latent period to pass and
found a 210% increased risk of developing breast cancer in young women (ie,
under the age of 35) who took OCPs for more than 10 years, if they began taking
them before the age of 18 [RR=3.1 (1.4-6.7)].
Q-Q: The studies you cited involved women who were less than 45 years old from data taken after 1980. What will happen to the risk of developing breast cancer for these women as they grow older?
No one knows. It would be wise
to learn from history. In the late 1940s an artificial female hormone named DES
(Diethylstilbestrol) was given to women to prevent miscarriages. For more than
25 years researchers maintained that DES use did not increase the risk of
breast cancer in women who took it. Finally, in the 1980s, it was discovered
that DES use increased breast cancer risk by about 35% — especially in older
women [30]. A similar phenomenon may be occurring with OCPs. The truth is, no
one knows how dangerous OCP use will be for women as they grow older.
Q-R: It has been noted that OCPs reduce the rate of uterine and ovarian cancer. Is this true?
Yes,
it is true. However it must be noted that OCPs also increase the risk of
cervical and liver cancer [31, 32, 33]. For example the largest study to date,
performed by the World Health Organization,
examined over 2,300 women and found that use of OCPs before the age of
25 increased the risk of invasive cervical cancer by 45% [34]. In addition,
more women get breast cancer in the U.S. than all of the other alluded to
cancers combined, making this the most dangerous risk in Western countries.
Oral contraceptives may be particularly risky in Asian and African countries where cervical and liver cancer are
prevalent [34, 35, 36].
Q-S: Often women who have painful menstrual cycles are placed on OCPs. Are there medical alternatives with less risks than OCPs?
Menstrual cramps can be
controlled by less harmful drugs than OCPs.
For example, taking 1,000 mg of Calcium and 399 mg of Magnesium around
the time of a woman’s onset of menstrual bleeding appears to help with
menstrual cramps and migraine headaches.
In addition, taking high dose anti-inflammatory agents (eg, ibuprofen) after one’s menstrual flow has started
(and under a doctor’s care) will often give relief. Also, the Journal of Adolescent Medicine published
a case report of a young lady who experienced a 90% reduction in her cramping
symptoms when taking Nicardipine after her
menstrual cramps had begun [37].
Nicardipine is a type of calcium channel blocker that is used for
treating hypertension.
Q-T: What about the risk
of “low dose” progestin containing contraceptives such as “the minipill,” or
long-acting progestins such as Norplant or Depo-Provera?
Skegg et al [38] pooled the
data from the World Health Organization (WHO) and New Zealand studies, the two largest studies that looked at women
who took Depo-Provera (active ingredient is DMPA: depot-medroxyprogesterone
acetate) for long periods of time. He found that women who had taken DMPA for
between 2 and 3 years before the age of 25 had a 310% statistically significant risk of getting breast cancer [RR=4.1 (1.6-10.90)] whereas women who
had taken DMPA for more than 3 years prior to the age of 25 had at least a 190% increased risk, that was also
significant [RR=2.9 (1.2-7.1)]. The
risks for long-term Norplant use in young women could be just as high as for
Depo-Provera users, although widespread tests have not been done because
Norplant was developed later than Depo-Provera. In regard to the progestin
containing “minipill,” the Oxford study noted an overall increased risk of 19%
(ie, RR=1.19 [0.89-1.49]) in women
who had taken minipills for 4 or more years, but they said nothing about
extended use in young women, especially women who took them prior to their FFTP
[17, p.98S]. The latter group of women might be at an especially increased
risk.
Q-U: How do the natural means of regulating birth compare to the artificial means?
Several well-designed trials
by the World Health Organization have shown that Natural Family Planning (NFP)
(ie, methods for determining when a woman is most fertile or infertile, based
on qualitative observations of cervical mucus and, for some NFP methods,
measuring basal body temperature) has had an effectiveness rate when used
correctly that is better than OCPs, that is, less than a 3% rate of pregnancies
per year. These trials have been done in both modern and less advanced
countries and have shown low annual pregnancy rates: the United Kingdom — 2.7%
[39], Germany — 2.3% [40], Belgium — 1.7% [41], and India — 2.0% [42]. One of the largest trials (of 19,843 women
performed by the World Health Organization in India) showed the failure rate to
be 0.2 pregnancies per 100 women yearly — a rate that is significantly
better than almost all artificial methods of contraception [43]. (For more
information regarding NFP see end of bibliography).
Q-V: How can the above noted information be verified?
Go to the nearest medical
library — nearly every hospital has one — and ask the librarian to help look up
the medical references of interest.
Q-W: What are the three questions never answered by the Harvard study?
The researchers at Harvard
have never answered the following simple questions:
1) How many women were there in the group who were under the age of 45 and who used OCPs for 5 years or more prior to their first full-term pregnancy (FFTP) (see page 69, Table 3 of your paper [ie, the women who were followed for 9,741 person-years]). What was the mean age for the women in this group?
2) How many women were there in the group who were under the age of 45 and never used OCPs? (see Table 2 page 68, these women were followed for 176,306 person-years). What was their mean age?
3) How many women were in the group who were under the age of 45 and had used OCPs for 10 years or more of total duration? (see Table 2, p. 68, the group that had 21,760 person-years of follow-up)
References:
11. Kirschstein RL, et al. Infiltrating duct carcinoma of the mammary gland of a Rhesus monkey after administration of an oral contraceptive: a preliminary report. J Natl Cancer Inst. 1972; 48: 551-553.
12. Geil, et al. FDA studies of estrogen, progestogens, and estrogen/ progesterone combinations in the dog and monkey. J Toxicol Environ Health. 3: 1979.
13. Shubik P. Oral contraceptives and breast cancer: laboratory evidence. In: Interpretation of Negative Epidemiological Evidence for Carcinogenicity. IARC Sci Pub. 1985; 65; 33.
14. Kahn RH, et al. Effect of long-term treatment with Norethynodrel on A/J and C3H/HeJ mice. Endocrinology. 1969; 84: 661.
15. Weisburger JH, et al. Reduction in Carcinogen Induced Breast Cancer in rats by an anti-fertility drug. Life Sci. 1968; 7: 259.
16. Welsch CW, et al. 17B-Oestradiol and enovid mammary tumorigenesis in C3H/HeJ female mice. Br J Cancer. 1977; 35: 322.
17. Anderson TJ, Battersby S, et al. Oral contraceptive use influences resting breast proliferation. Hum Pathol. 1989; 20: 1139-1144.
18. Alderson Reporting Company. Transcripts of oral arguments before court on abortion case. New York Times. April 27, 1989; B12.
19. Brind J, Chinchilli M, et al. Induced abortion as an independent risk factor for breast cancer: a comprehensive review and meta-analysis. J Epidemiol Community Health. 10/ 1996; 50: 481-496.
10. Stewart DR, Overstreet JW, et al. Enhanced ovarian steroid secretion before implantation in early human pregnancy. J Clin Endocrinol Metab. 1993; 76: 1470-1476.
11. Norman RJ et al. Inhibin and relaxin concentration in early singleton, multiple, and failing pregnancy: relationship to gonadotropin and steroid profiles. Fertility and Sterility. 1993; 59: 130-137.
12. Pike MC, Henderson BE, et al. Oral contraceptive use and early abortion as risk factors for breast cancer in young women. Br J Cancer. 1981; 43: 72-76.
13. Wingo PA, Lee NC, et al. Age-specific differences in the relationship between oral contraceptives use and breast cancer. Cancer (supplement). 1993; 71: 1506-1517.
14. Chilvers C, McPherson K, et al. Oral contraceptive use and breast cancer risk in young women (UK National Case-Control Study Group). The Lancet. May 6, 1989: 973-982.
15. Brinton LA, Daling JR, et al. Oral contraceptives and breast cancer risk among younger women. J Natl Cancer Inst. 6/7/1995; 87: 827-35.
16. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. The Lancet. 1996; 347: 1713-1727.
17. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996; 34: S1-S106.
18. Rosenberg L, Palmer JR, et al. A case-control study of the risk of breast cancer in relation to oral contraceptive use. Am J Epidemiol. 1992; 136: 1437-44.
19. White E, Malone K, Weiss N,
Daling J. Breast cancer among young U.S. women in relation to oral
contraceptive use. J Natl Cancer Inst.
1994; 86: 505-514.
20. Thomas DB. Oral contraceptives and breast cancer: review of the epidemiologic literature. Contraception. 1991; 43: 597-643.
21. Romieu I, Berlin J, et al. Oral contraceptives and breast cancer. Review and meta-Analysis. Cancer. 1990; 66: 2253-2263.
22. Hankinson SE, et al. A prospective study of oral contraceptive use and risk of breast cancer (Nurses Health Study, United States). Cancer Causes and Control. 1997; 8: 65-72.
23. Beral V, et al. Mortality
associated with oral contraceptive use: 25 year follow up of cohort of 46,000
women from Royal College of General Practitioners’ oral contraception study. Br Med J. 318. 1/1999: 96-99.
24. Rookus MA, Leeuwen FE. Oral contraceptives and risk of breast cancer in women ages 20-54 years. The Lancet. 1994; 344: 844-851.
25. Weinstein A, Mahoney M, et al. Breast cancer risk and oral contraceptive use: results from a large case-control study. Epidemiology. 1991; 2: 353-358.
26. Palmer J, Rosenberg L, et al. Oral contraceptives use and breast cancer risk among African-American women. Cancer Causes and Control. 1995; 6: 321-331.
27. Thomas DB, Noonan EA. Breast cancer and combined oral contraceptives: results from a multinational study [The WHO collaborative study of Neoplasia and steroid contraceptives]. Br J Cancer. 1990; 61: 110-119.
28. Wang Q, Ross R, et al. A case-control study of breast cancer in Tianjin, China. Cancer Epidemiology. 1992; 1: 435-439.
29. Miller D, Rosenberg L, et al
Breast cancer before age 45 and oral contraceptive use: new findings. Am J Epidemiol. 1989; 129: 269-279.
30. Colton T, Greenberg ER, et al. Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. JAMA. 1993; 269: 2096-3000.
31. Thomas DB, et al. Oral contraceptives and invasive adenocarcinomas and adenosquamos carcinomas of the uterine cervix. Am J Epidemiol. 1996; 144: 281-289.
32. Ebeling K, et al. Use of oral contraceptives and risk of invasive cervical cancer in previously screened women. Int J Cancer. 1987; 39: 427-430.
33. Kenya PR. Oral contraceptive use and liver tumours: a review. East African Medical Journal. 1990. 67:146-153.
34. Thomas DB, et al. Invasive squamos-cell cervical carcinoma and combined oral contraceptives: Results from a multinational study. Int J Cancer. 1993; 53: 228-236.
35. Parkin, et al. Estimates of the worldwide frequency of sixteen major cancers in1980. Int J Cancer. 1988; 41: 184-197.
36. Fauci AS, et al. Harrison’s: Principles of Internal Medicine. 14th ed. New York: McGraw Hill; 1998.
37. Earl DT, et al. Calcium channel blockers and dysmenorrhea. Journal of Adolescent Medicine. 1992; 13: 107-108.
38. Skegg DCG, Noonan EA, et al. Depot medroxyprogesterone acetate and breast cancer (A pooled analysis of the World Health Organization and New Zealand studies). 1995; JAMA: 799-804.
39. Clubb EM, et al. A pilot study on teaching NFP in general practice: current knowledge and new strategies for the 1990s. Washington, D.C.: Georgetown University; 1990: 130-132.
40. Frank-Hermann P, et al. Effectiveness and acceptability of the symptothermal method of NFP in Germany. Am J Obstet Gynecol. 1991; 165: 2045-2052.
41. De Leizaola MA. De premiere d’une etude prospecive d’efficacite du planning famillial naturel realisee en Belgique francophone. J Gyncol Obstet Biol Rev. 1994; 23: 359-364.
42. DorairajK. The modification mucus method in India. Am J Obstet Gynecol. 1991; 165: 2066-2067.
43. Ryder RE. “Natural Family Planning”: effective birth control supported by the Catholic Church. Br Med J. 1993; 307: 723-726.
For more information on NFP call or write to:
The Couple to Couple League 1-513-471-2000
Pope Paul VI Institute 1-402-390-6600
Family of the Americas 1-800-443-3395
Billings Ovulation Method Association 1-888-637-6371
The St. Augustine Foundation 1-877-554-4637
NW Family Services 1-503-215-6377